Could advancements in gene sequencing technology accelerate scientific breakthroughs and provide attractive investment opportunities for years to come?

Key Points
- Technological advancements have boosted the speed of sequencing, enhanced its accuracy and lowered costs meaningfully.
- We believe the total addressable market for gene sequencing is poised to grow an average of 10% to 20% per year over the next 15 years.
- In our view, significant demand for sequencing tests should more than offset pricing pressures and may ultimately lead to the continuation of high growth in the sequencing market through 2040.
The human genome is the playbook by which the human body functions. As such, the ability to read and understand the human genome is imperative to research on human health and disease management. Current in-depth knowledge about the human body—which is considerable, yet by no means complete—is largely attributable to the introduction and evolution of deoxyribonucleic acid (DNA) sequencing technology.
After years of dramatic expansion in this revolutionary space, bearish investors are concerned that the genetic sequencing market is nearing saturation and that growth is reaching a plateau, particularly as innovation and competition drive prices lower. However, gene sequencing technology continues to advance at a brisk pace. Additionally, the demand for DNA sequencing information continues to climb, and a myriad of new applications are still in nascent stages of development. Backed by the careful and comprehensive analysis of our multidimensional research platform, we believe the total addressable market for gene sequencing is poised to grow by an average of 10% to 20% per year over the next 15 years. In our view, this cutting-edge market could provide scientific and medical breakthroughs, as well as attractive investment opportunities, for years to come.
What Is Genetic Sequencing?
Our complete set of DNA is referred to as our genome. Found in nearly every cell of a human body, DNA carries the instructions for synthesizing proteins, which are responsible for all the ways in which our bodies develop and function. Through a process called transcription, the genetic code stored in DNA is transferred into ribonucleic acid (RNA). RNA, which acts as DNA’s messenger, carries that code to an area of the cell that serves as a protein building-block factory, and through a process called translation, proteins are created.
A DNA molecule is comprised of two adjoining strands that form a long spiral, or the familiar double-helix structure. These strands, which are linked together by hydrogen bonds, each contain a combination of four chemical building blocks, or bases—adenine (A), cytosine (C), guanine (G) and thymine (T). These bases exist in pairs, with A always pairing with T and C always pairing with G. The sequence of these base pairs dictates the genetic information that a DNA molecule carries.
DNA sequencing is the process by which scientists read the exact order of base pairs across a DNA segment or an entire genome.[1] To do this, scientists take a DNA sample (of blood, for instance) and put it through a sequencer, which is the instrument used to read the sample. The sequencer produces an output that appears as a string of As, Cs, Gs and Ts, corresponding to each particular base’s position in the DNA strand. As bases exist in pairs, sequencers only need to read one strand of the DNA molecule.
Scientists can take a DNA sequencing readout and compare it to a “normal” reference genome to determine how it differs and if those differences are causing specific diseases or conditions. Today, sequencers are used in many different types of laboratories, ranging from small academic research labs and large core research labs to clinical labs located in hospitals.
Revolutionary Advancements in Gene Technology
DNA sequencing technology is not new; early iterations can be traced back to the 1970s. However, in its earlier stages, sequencing was slow, expensive and far less accurate than it is today. More recently, technological advancements have boosted the speed of sequencing, enhanced its accuracy and lowered costs meaningfully—by over 100,000 times since 2001.[2]
Much of this improvement can be attributed to market leader Illumina. By 2014, Illumina had established a foothold in the market, securing 70% of the market share and generating 90% of all DNA data produced globally.[3] Today, while Illumina remains a dominant force, owing to its continual innovation and ability to target different segments of the industry, several other viable competitors have emerged. These newer participants are focused on various factors, including innovative new software tools and newer products that reduce the costs of sequencing. Continued competition in the space may pressure sequencing prices to decline further and lead to more cost-efficient and sustainable technological improvements.
DNA Sequencing Applications
As gene sequencing has progressed, its range of applications has significantly expanded—and many of the applications today would have been unfeasible at the pace, cost and accuracy of sequencing technology years ago. Currently, researchers and clinicians are using DNA sequencing for a number of applications, including discovery and translational research, clinical trials, population health, newborn screening and molecular diagnostics.
Gene Sequencing Tests by Use Case (2022)
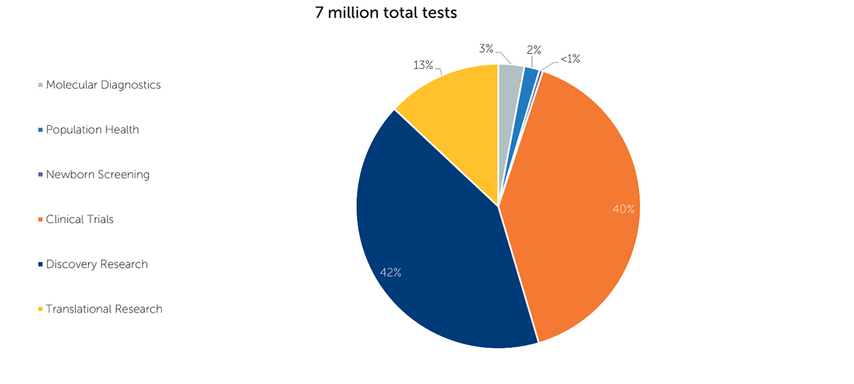
Some applications are much more established than others; however, with continued advancements in the field over time, we expect the makeup of the market to shift dramatically over the next 15 years.
Today, clinical trials and discovery research comprise the lion’s share of the DNA sequencing market. In contrast, emerging applications like molecular diagnostics make up less than 4% of all sequencing tests run today. We believe that the established sequencing market should continue to grow, but the emerging applications should grow at a much higher pace. Over the next 15 years, we may see a shift in the market where areas like discovery research and clinical trials no longer make up the majority of sequencing volumes. These more mature markets may grow at a slower pace than they have done historically, but we believe the newer use cases should more than offset this slowdown, thus driving the overall sequencing market growth.
Newton’s Sequencing Model
The DNA sequencing market size estimates and models available today are typically expressed in dollar amounts. However, it is also beneficial to consider what could happen to the sequencing test volume as the technology continues to advance, and what the demand may be if price was not a factor. To do so, we leveraged the expertise of our multidimensional research platform—particularly our private markets, investigative research and research associates teams—to build our own model.
The model we constructed forecasts the volume of gene sequencing tests that could be demanded each year through 2040. It also breaks down these volumes by application. Members of our private markets team identified innovative private companies in the space and met these companies to better understand what the market is currently missing and what their specific technologies aim to improve. Our investigative research team, comprising former investigative journalists, sought researchers and clinicians using sequencers in their day-to-day work. They interviewed them to explore how they are using the instruments, how much more they might use the instruments if prices were to drop further, and their chief complaints about the technology. Our research associates helped us compile the information and create a dynamic model incorporating multiple inputs for each specific use case.
Conclusion
DNA sequencing has reached the point where cost is no longer an inhibiting factor and the accuracy and speed of the instruments on the market have significantly improved. Today, running DNA sequencing tests is efficient, and most importantly, the outcomes are useful and reliable.
As genetic sequencing technology continues to advance over time, we believe the uptake of the technology should continue to increase across the market. This has the potential to play a crucial role in understanding human health and disease progression. In our view, significant demand for sequencing tests should more than offset pricing pressures and may ultimately lead to the continuation of high growth in the sequencing market through 2040.
Sources:
[1] National Human Genome Research Institute. Updated November 6, 2023. https://www.genome.gov/genetics-glossary/Base-Pair#:~:text=%E2%80%8BBase%20Pair&text=Attached%20to%20each%20sugar%20is,and%20cytosine%20pairs%20with%20guanine.
[2] Front Line Genomics. July 26, 2021. https://frontlinegenomics.com/a-brief-history-of-next-generation-sequencing-ngs/
[3] MIT Technology Review. June 15, 2015. https://www.technologyreview.com/lists-tr50/50-smartest-companies-2014/intro#Illumina
PAST PERFORMANCE IS NOT NECESSARILY INDICATIVE OF FUTURE RESULTS. Any reference to a specific security, country or sector should not be construed as a recommendation to buy or sell this security, country or sector. Please note that strategy holdings and positioning are subject to change without notice. The securities mentioned are only for illustrating the investment process of Newton Investment Management. These opinions should not be construed as investment or any other advice and are subject to change. This material is for information purposes only and does not constitute an offer or solicitation to invest. For additional Important Information, click on the link below.
Important information
For Institutional Clients Only. Issued by Newton Investment Management North America LLC ("NIMNA" or the "Firm"). NIMNA is a registered investment adviser with the US Securities and Exchange Commission ("SEC") and subsidiary of The Bank of New York Mellon Corporation ("BNY Mellon"). The Firm was established in 2021, comprised of equity and multi-asset teams from an affiliate, Mellon Investments Corporation. The Firm is part of the group of affiliated companies that individually or collectively provide investment advisory services under the brand "Newton" or "Newton Investment Management". Newton currently includes NIMNA and Newton Investment Management Ltd ("NIM") and Newton Investment Management Japan Limited ("NIMJ").
Material in this publication is for general information only. The opinions expressed in this document are those of Newton and should not be construed as investment advice or recommendations for any purchase or sale of any specific security or commodity. Certain information contained herein is based on outside sources believed to be reliable, but its accuracy is not guaranteed.
Statements are current as of the date of the material only. Any forward-looking statements speak only as of the date they are made, and are subject to numerous assumptions, risks, and uncertainties, which change over time. Actual results could differ materially from those anticipated in forward-looking statements. No investment strategy or risk management technique can guarantee returns or eliminate risk in any market environment and past performance is no indication of future performance.
Information about the indices shown here is provided to allow for comparison of the performance of the strategy to that of certain well-known and widely recognized indices. There is no representation that such index is an appropriate benchmark for such comparison.
This material (or any portion thereof) may not be copied or distributed without Newton’s prior written approval.
In Canada, NIMNA is availing itself of the International Adviser Exemption (IAE) in the following Provinces: Alberta, British Columbia, Manitoba and Ontario and the foreign commodity trading advisor exemption in Ontario. The IAE is in compliance with National Instrument 31-103, Registration Requirements, Exemptions and Ongoing Registrant Obligations.




