Disruptive innovation in health care could help us age more healthily
For the first time in human history, the number of over-65s in the world has overtaken the number of under-5s. The ageing global population is one of the most significant transformations of the 21st century, and as the chart below shows, this change is accelerating.
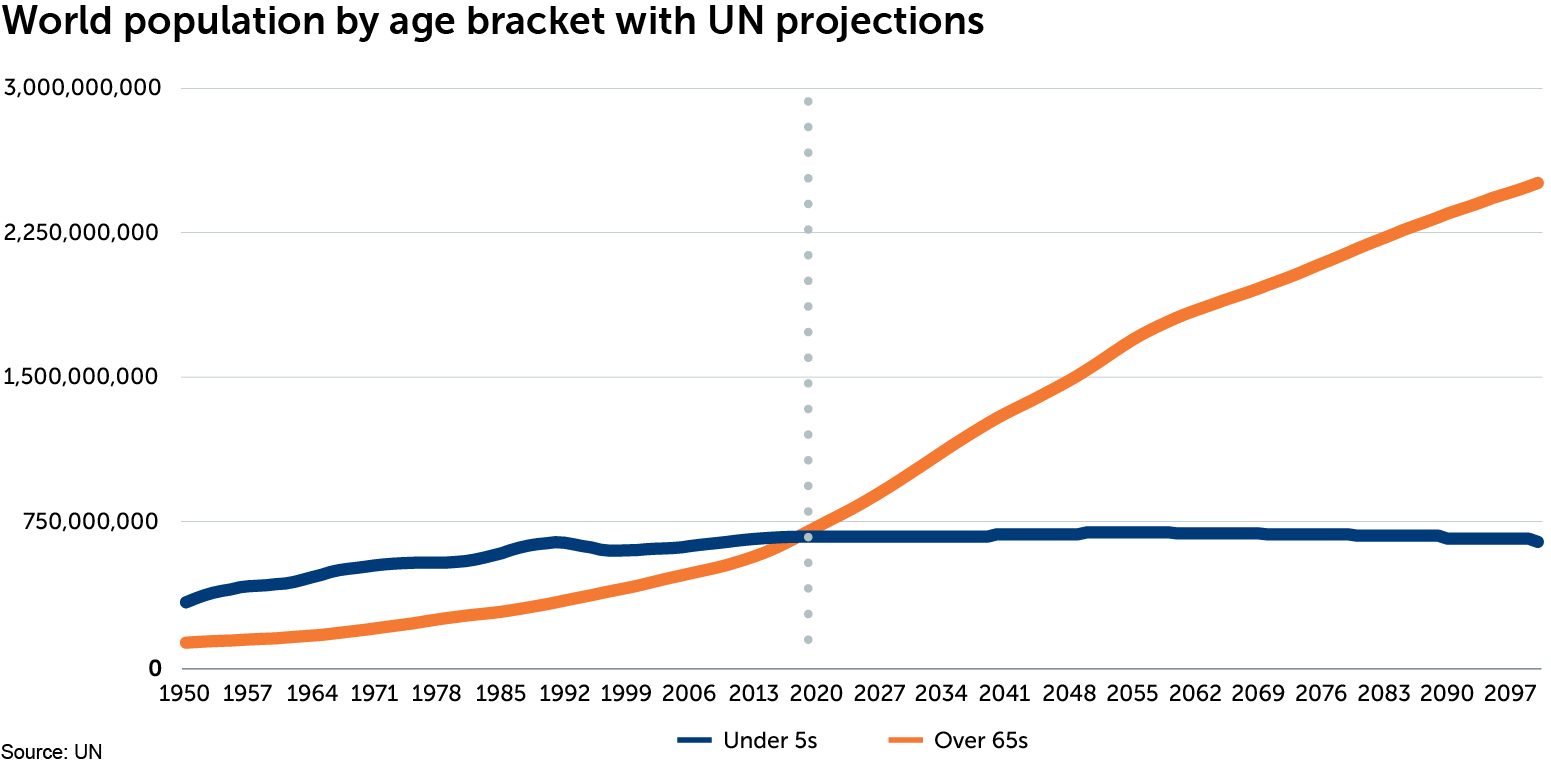
Our ‘healthy demand’ investment theme highlights how ageing populations combined with severe budgetary constraints in mature economies, and growing incomes in the developing world, are driving demand for affordable health-care solutions. Although people are living longer, many spend their later years in poor health, or suffering from a disability. This has profound implications for the labour market, productivity and the quality of life of affected individuals and their families. It also has huge implications for the health-care industry. As the ranks of over-65s expand, demand for affordable interventions to either delay the onset of poor health and disability and/or to improve the quality of life and ability to function, will increase. We are now seeing disruptive innovation in health care lead to the development of treatments which aim to cure, or delay the onset of, several common age-related conditions.
Eye drops could replace glasses
As we age, the lenses in our eyes start to harden. This leads to presbyopia, or long-sightedness. Novartis has an interesting drug in Phase 2 development, called UNR844, which aims to slow or even halt this hardening of the lens with a couple of eye drops taken twice a day. It is too early to be certain, but UNR844 may be able to reverse the formation of cataracts, the leading cause of blindness.
Improving the efficacy of vaccines
Our immune system weakens as we age. As a result, the over-65s are more at risk of suffering severe consequences, or even dying, from influenza. Annual vaccinations are a useful way to reduce the likelihood of catching influenza but they are not faultless. Today’s egg-based vaccine manufacturing process has been used more or less unchanged for around 70 years. Each year, over 500 million hen’s eggs are delivered to vaccine manufacturers. Each egg is injected with the influenza virus. Then the egg is incubated while the virus grows. Finally, an egg cutter removes the top of each shell and a probe extracts the liquid. Most vaccines contain four strains of the influenza virus, so each vaccine needs four eggs. However, lab-grade eggs require heavy doses of antibiotics to guarantee their safety, which we need to reduce given increasing antibiotic resistance.
Given it takes time to lay 500 million eggs and grow vaccines in them, the World Health Organisation (WHO) has to predict which influenza strains to protect against six months in advance. The WHO hedges its bets by choosing three or four strains rather than one, but predicting anything six months in advance is inevitably going to be hit and miss. Unfortunately, viruses can also mutate inside the egg so they may not always align with the exact influenza strain in circulation. In 2018, for example, vaccine effectiveness was only 38%.[1]
In response, some companies, including CSL, are working on cell-based vaccine-manufacturing processes which have the potential to offer superior protection versus egg-based vaccines, owing to them being a closer match to the virus in circulation. We only have a few years of data, so it is too early to know for sure, but early evidence suggests that not only is this new technology scalable, it is also more effective.
Improving cancer diagnostics
Several companies are working on developing blood tests designed to detect the early signs of cancer. A company called GRAIL released promising clinical trial results earlier in 2019. According to GRAIL’s data, its next-generation sequencing blood test was able to detect a strong signal for 12 cancer types at very early stages correctly between 60-90% of the time, depending on the type of cancer. Importantly, the false-positive rate – identifying someone with cancer who did not actually have it – was just 1%. In May 2019, GRAIL secured ‘breakthrough device designation’ (a fast-track approval process) from the US Food and Drug Administration for its blood test.
[1] Source: CDC
This is a financial promotion. These opinions should not be construed as investment or other advice and are subject to change. This material is for information purposes only. This material is for professional investors only. Any reference to a specific security, country or sector should not be construed as a recommendation to buy or sell investments in those countries or sectors. Please note that holdings and positioning are subject to change without notice.






Comments